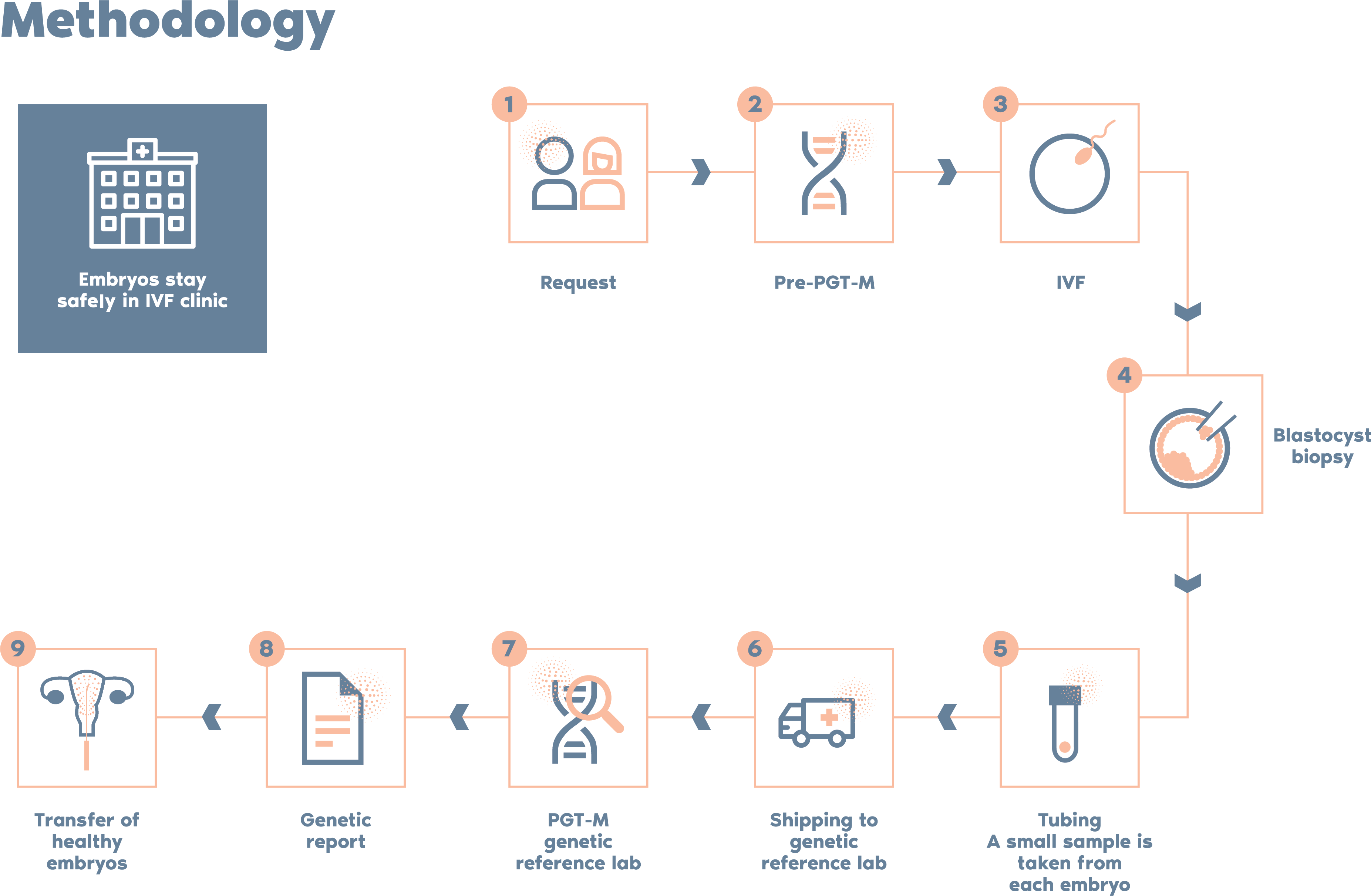
What is the procedure?

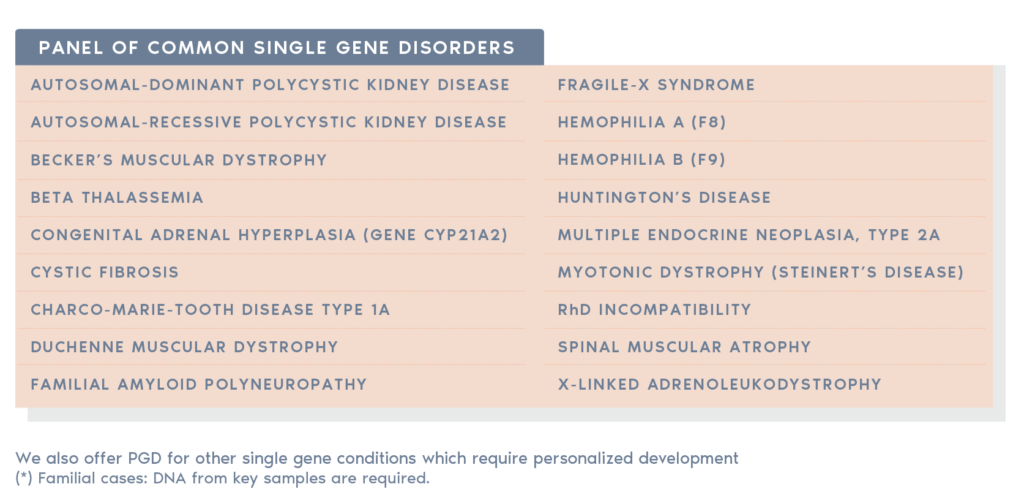
For clinics in the UK, a condition must be approved by the Human Fertilisation & Embryology Authority (HFEA) to perform PGT-M. Igenomix are able to support clinics in their applications for additions to this list. Note that this restriction does not apply to non-UK clinics working with Igenomix UK.
Title: The clinical utility of PGD with HLA matching: a collaborative multi-centre ESHRE study
Authors: Kakourou G, Kahraman S, Ekmekci G C, Tac H A, Kourlaba G, Kourkouni E, Cervero Sanz A, Martin J, Malmgren H, Giménez C, Gold V, Carvalho F, Billi C, Chow J F C, Vendrell X, Kokkali G, Liss J, Steffann J, Traeger-Synodinos J
Publication: Hum Reprod. 2018 Mar 1;33(3):520-530. doi: 10.1093/humrep/dex384.
PMID: 29432583
Authors: De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C
Publication: Hum Reprod. 2017 Oct 1;32(10):1974-1994. doi: 10.1093/humrep/dex265.
PMID: 29117384