What test does the EndomeTRIO analysis include?
ERA
The ERA test evaluates the receptivity of the endometrium to determine if, at the time of biopsy, the uterine lining is receptive to embryo implantation. This information allows us to determine the ideal time for a personalised embryo transfer (pET). The ERA test resulted in a 73% pregnancy rate in patients with implantation failure.
ALICE
ALICE (Analysis of Infectious Chronic Endometritis) is a diagnostic test to detect the bacteria causing chronic endometritis (CE), a condition that is associated with infertility.
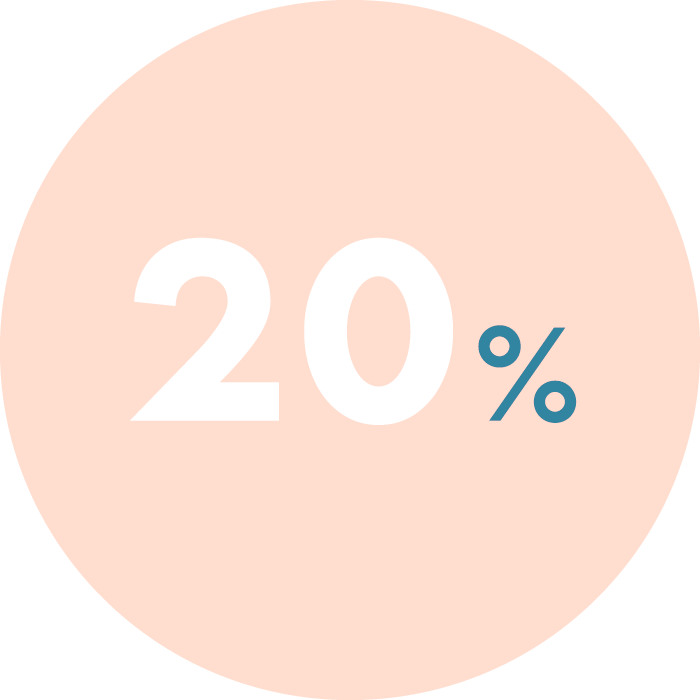
Independent studies show that this condition affects up to 30% of infertile patients, and in cases of repeated implantation failure or recurrent pregnancy loss, this can rise to 66%*.
ALICE identifies the most common bacteria causing CE and helps clinicians to recommend appropriate antibiotic and probiotic treatments.
EMMA
EMMA (Endometrial Microbiome Metagenomic Analysis) is a screening test to evaluate the endometrium at the microbiological level, improving clinical management of infertile patients.
By analysing the endometrial microbiome, EMMA determines whether the uterine microbial environment is optimal for embryo implantation.
EMMA provides information about the ten most abundant bacteria in the endometrium, recommending embryo transfer or appropriate treatment, depending on results.
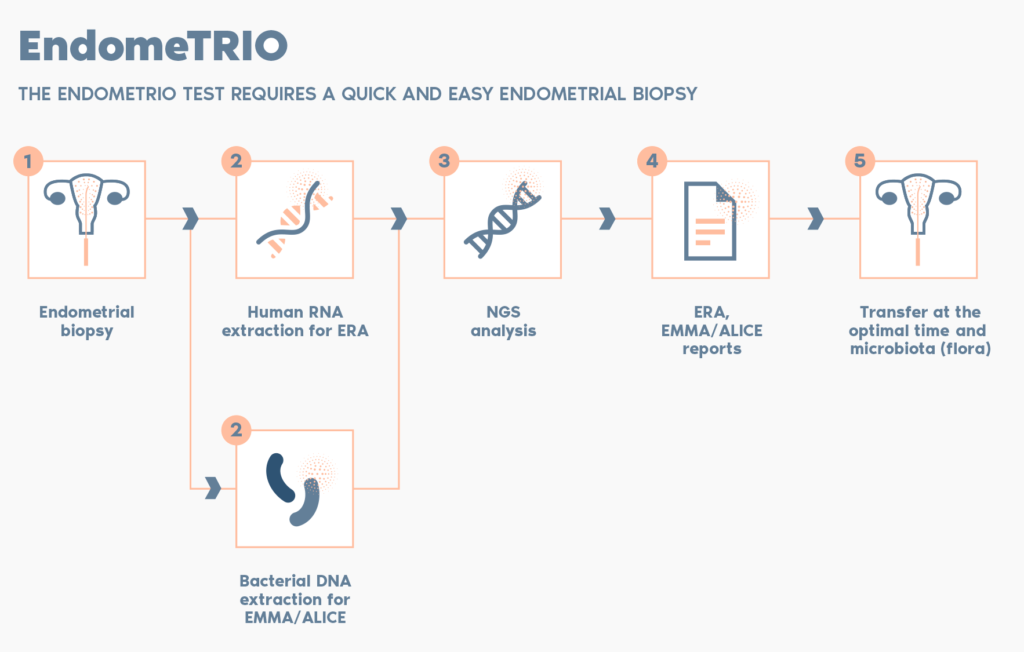
What is the procedure?