What is ALICE test?
ALICE is a test that detects pathogenic bacteria and recommends adequate treatment.
Pathogenic bacteria cause chronic endometritis, which is linked to implantation failure and recurrent miscarriage.
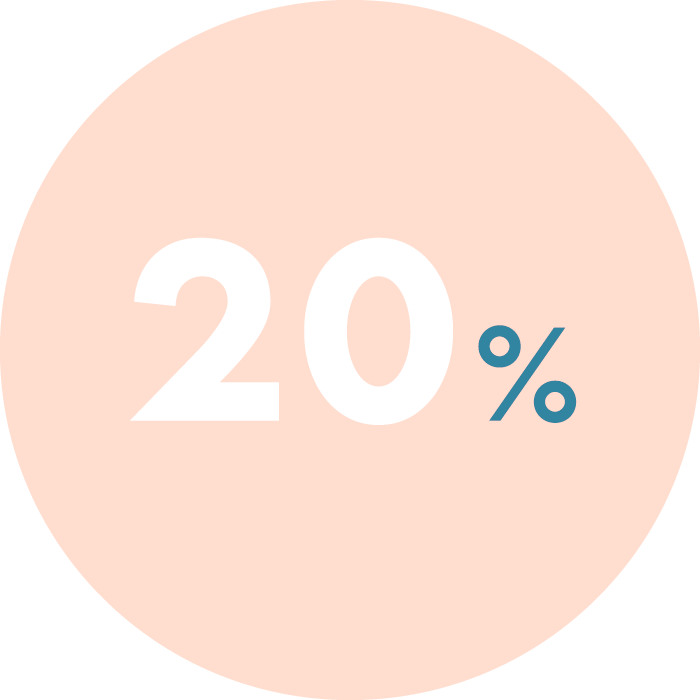
In cases of repeated implantation failure or recurrent pregnancy loss, chronic endometritis can rise to 66%.
Methodology
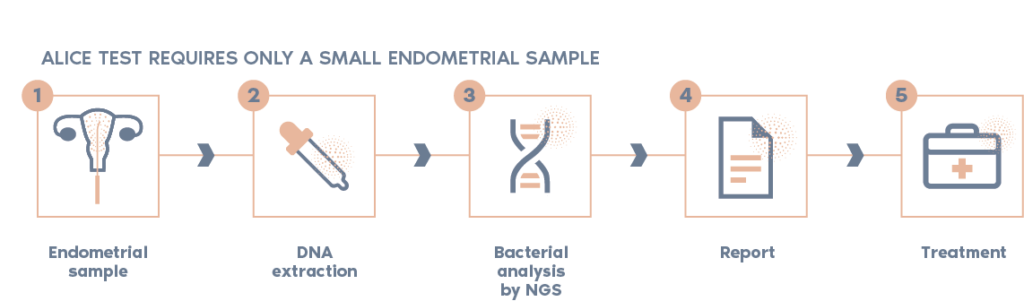
ALICE can be performed on a small piece of an endometrial biopsy.
If the patient is undergoing an ERA test, a small portion of the same biopsy can be used, so no additional sample will be required.
How to collect and send the sample
For EMMA, an endometrial biopsy should be taken from day 15 to day 25 of the menstrual cycle. After retrieval, it is important that the sample is briefly shaken vigorously and immediately placed in the refrigerator at 4–8°C for a minimum of 4 hours.