What is the EMMA test?
EMMA is a test that analyses the endometrial microbiome to help identify abnormalities associated with a poor reproductive prognosis.
EMMA indicates the endometrial microbiome balance, providing information on the proportions of healthy endometrial bacteria, including those linked to higher pregnancy rates. EMMA also includes ALICE, which detects pathogenic bacteria that can cause chronic endometritis.
Methodology
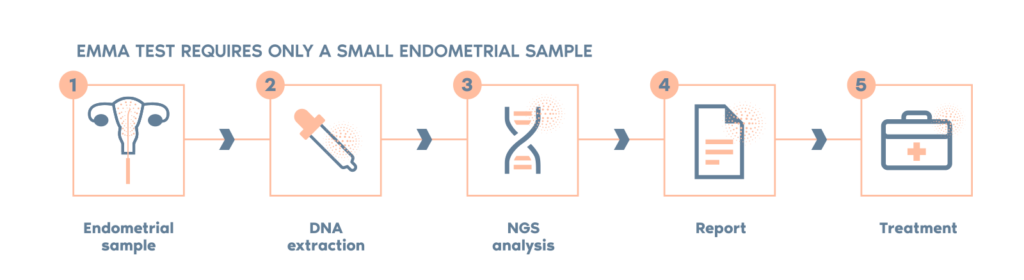
- EMMA can be performed on a small sample of an endometrial biopsy.
- If the patient is undergoing an ERA test, a small portion of the same biopsy can be used, so no additional sample will be required.