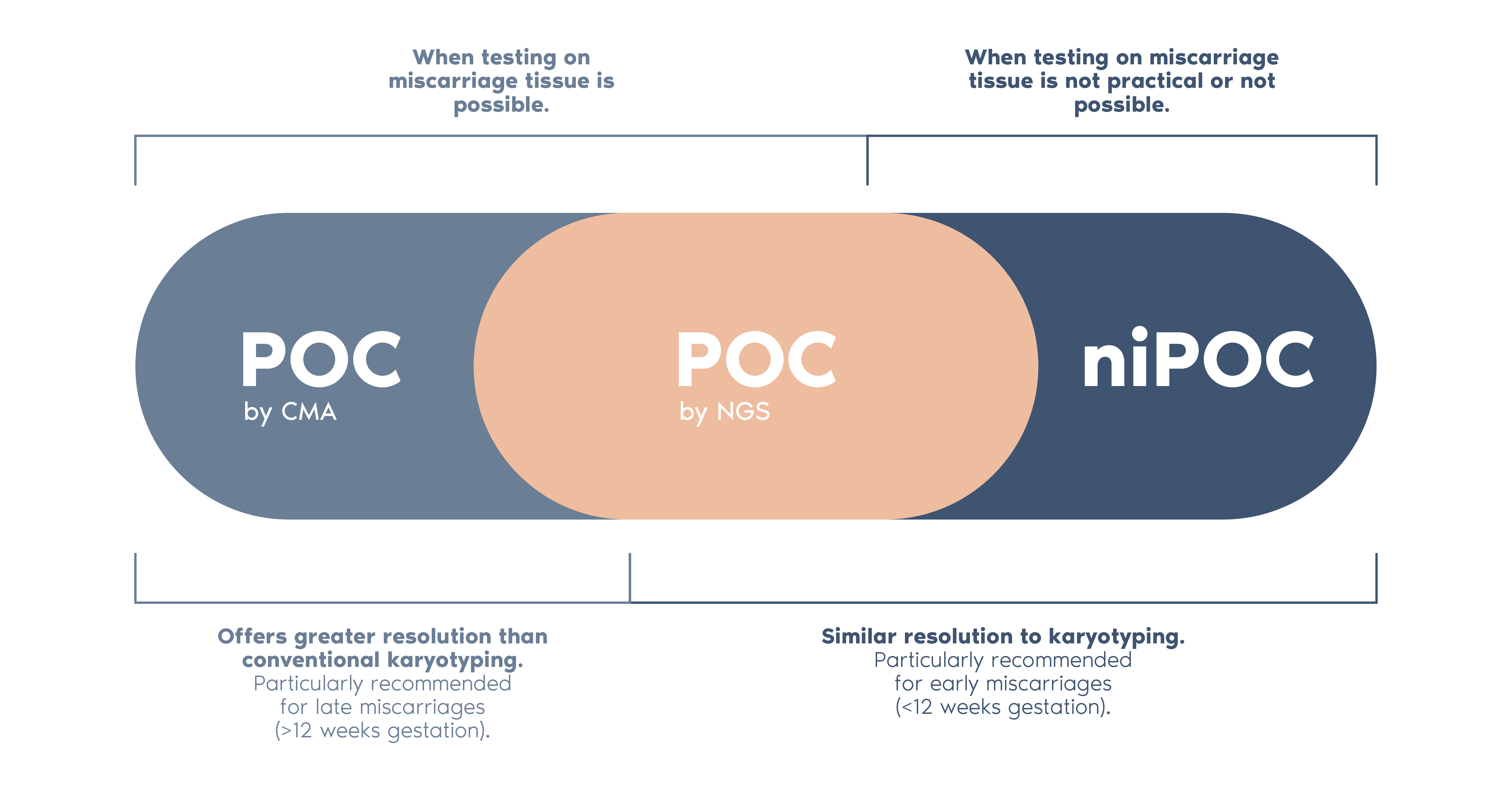
Our POC portfolio consists of:
- POC by Next Generation Sequencing (NGS)
- Non-invasive POC (niPOC)
- POC by Chromosomal Microarray (CMA)
What is POC testing?
Our portfolio of POC tests analyse whether a miscarriage may have been caused by a
chromosomal abnormality.
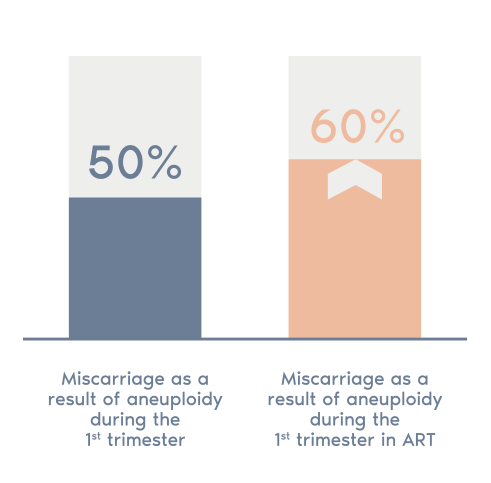
- In around 50% of first trimester miscarriages a chromosome abnormality is present.
- This figure rises to 60% in cases of assisted reproductive treatment and further increases with age.
- The POC test analyses the chromosomes and is able to identify alterations to the number of chromosomes (aneuploidies) as well as losses and gains of chromosome fragments.
- For POC (by CMA and by NGS) it is possible to identify some types of ploidy alterations (loss or gain of a complete set or sets of chromosomes) and determine the origin of the tissue under study (foetal or maternal).
How is the POC test performed?
There are different methods depending on the test carried out:
niPOC Methodology
**It is essential that the blood sample is collected before the evacuation of the foetal tissue takes place. If pharmacological treatment is being used for the evacuation, the blood should be collected prior to taking the medication.
POC Methodology
For POC by NGS and POC by CMA