What is EMBRACE?
- EMBRACE is a non-invasive test to prioritise embryos for transfer that avoids invasive embryo biopsy; potentially increasing accessibility for a wider patient population.
- EMBRACE scores embryos according to their probability of being healthy (euploid) and viable based on chromosomal analysis.
- The recent identification of embryo cell-free DNA in spent blastocyst media opened a new era of possibilities for non-invasive embryo aneuploidy testing in assisted reproductive technologies.
- During in-vitro embryo development, embryo cell-free DNA is released into the culture medium, with higher concentrations as the number of cells increases at blastocyst stage.
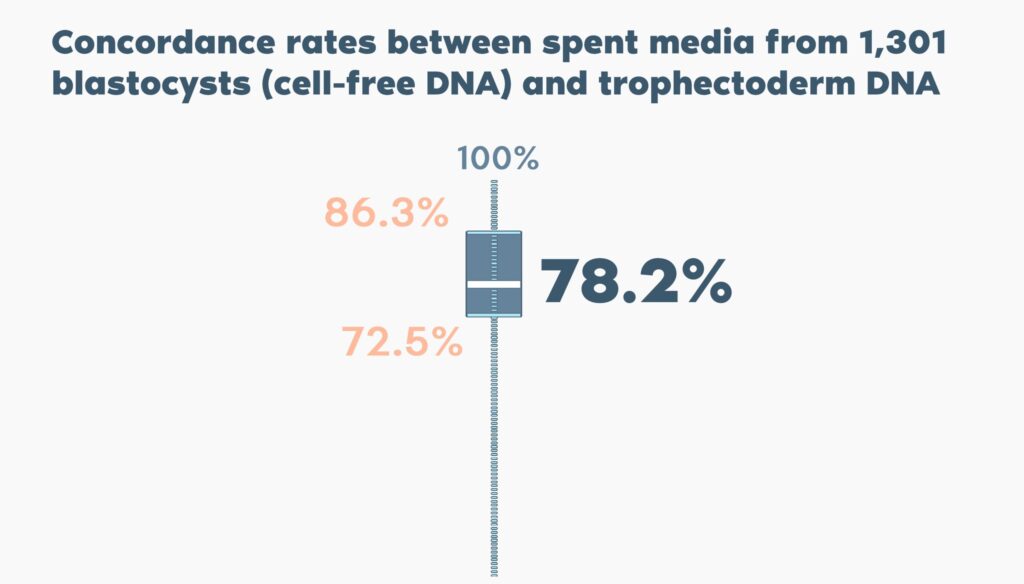
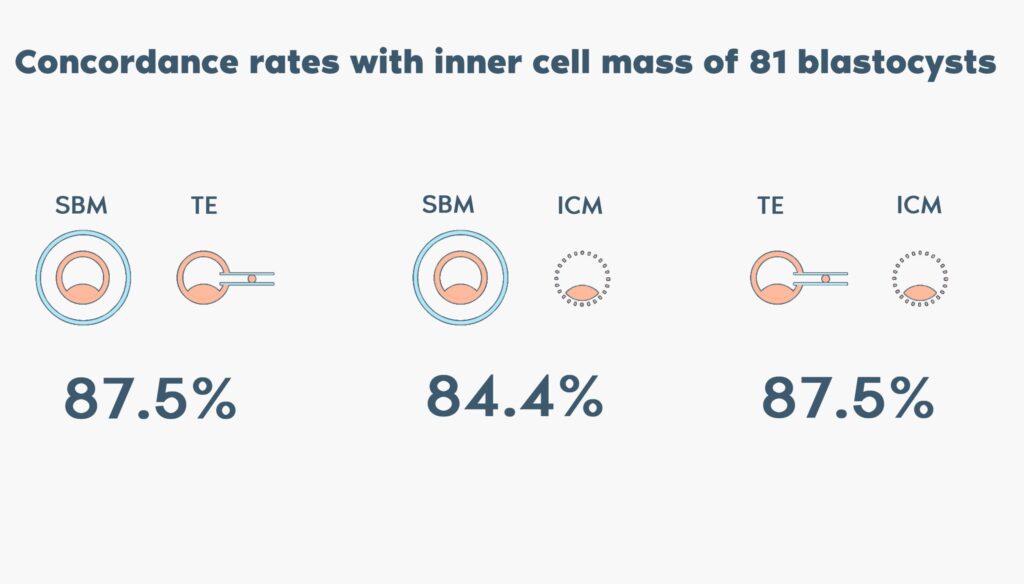
- Spent blastocyst medium containing cell-free DNA from the embryo can be analyzed using next generation sequencing (NGS), representing a non-invasive approach to estimate the chromosome copy number of the blastocyst without the need for trophectoderm biopsy.
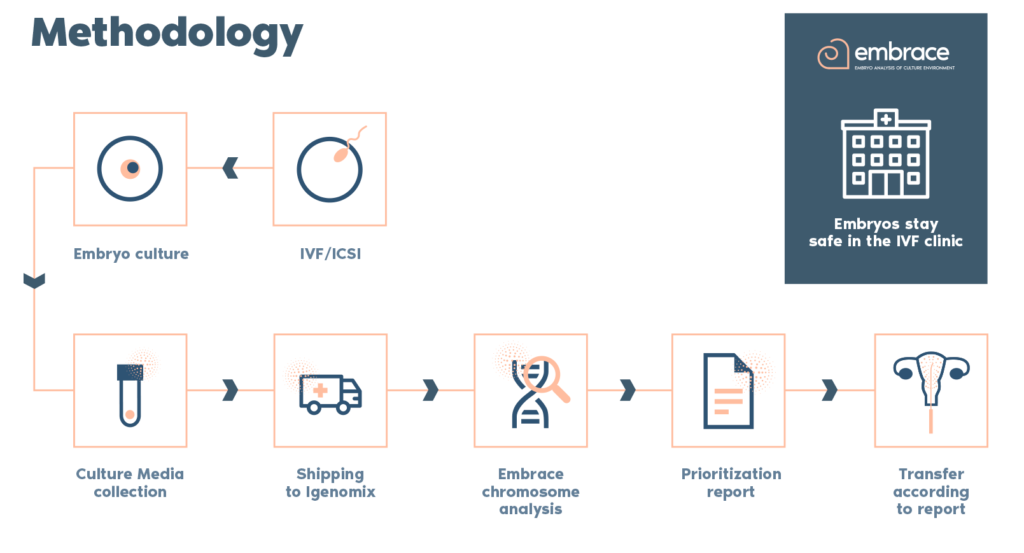
What is the procedure?