-
Repeat expansions are common genetic variations that are usually associated to neurogenetic disorders. These types of mutations are unstable and dynamic, and the number of repeats can change from generation to generation.
-
Conditions that are caused by repeat expansions are clinically and genetically heterogeneous and vary depending on the repeat sequence, size and location within the disease gene, and whether the repeat is translated into protein.
-
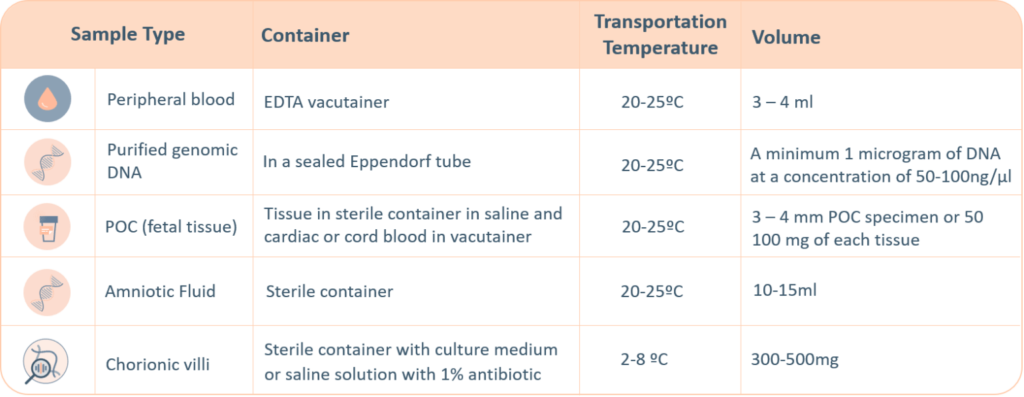
PCR-based analysis of repeat lengths is the most cost-effective method for the diagnosis of diseases associated with expansion repeats.
EXP – Expansion Repeats
For polynucleotide expansions in a gene or genomic region.